Abstract
Introduction
Up to 20% of MM patients present with light-chain-only multiple myeloma (LCMM), which is not detectable using serum protein electrophoresis testing. 24-hour urinalysis may be the guideline-endorsed method for monitoring LCMM, but impracticalities can limit its use. We sought to assess how patients with MM and LCMM were followed for progression in the community setting by specimen type using real-world data, analyzing the frequency of testing for patients with MM (based on SPEP) or LCMM (based on UPEP or SFLC).
Methods
This retrospective cohort study used information from the Flatiron Health database, which is derived from electronic health record data; Institutional Review Board approval with a waiver of informed consent was obtained. The cohort included 4591 patients followed for MM and LCMM from 01/01/2011 to 06/30/2018 at community oncology clinics across the United States. The cohort was limited to patients with a confirmed diagnosis of MM on or after 01/01/2011, who received at least one line of therapy and did not receive a stem cell transplant. Patients whose start of MM treatment (captured through chart review) was > 30 days before the start of structured activity in the database were excluded as this may indicate missing therapy data. The specimen type was the test used to follow patients for progression throughout their disease course. Tests were eligible to define the specimen type if they occurred ≤ 90 days before or any time after an initial MM diagnosis. If a patient had multiple test types within this timeframe, the specimen type was determined using a hierarchy (at least one detectable SPEP [≥ 1 g/dL] > at least one detectable 24-hour UPEP [≥ 200 mg] > SFLC [any numeric value]), consistent with International Myeloma Working Group (IMWG) criteria. A LCMM diagnosis was assumed when a detectable M spike (≥1 g/dL) via SPEP could not be found 90 days before or any time after the initial MM diagnosis date, but a detectable lab value was found within that timeframe via 24-hour UPEP (≥ 200mg) or a SFLC (any numeric value).
Results
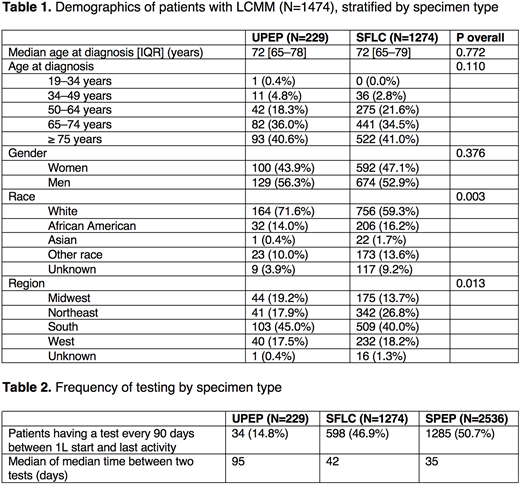
Among all patients in the cohort with MM (N=4591), 27.7% (N=1272) received a UPEP test, 58.1% (N=2666) received a SFLC test, and 79.2% (N=3635) received a SPEP test at baseline, defined as occurring within 90 days on either side of the MM diagnosis date. Patients with a UPEP specimen type had less frequent testing than patients with a SPEP or SFLC specimen type. For patients with a UPEP specimen type (N=229), the median of the median time between two tests was 95 days, compared to 42 days for patients with a SFLC specimen type (N=1274) and 35 days for patients with a SPEP specimen type (N=2536). 32.7% (N=1503) of the cohort had LCMM; of those patients, 84.8% were followed by SFLC while only 15.2% were followed by UPEP.
Conclusions
24-hour urine tests for UPEP are not used often in routine clinical practice despite IMWG guidelines: the guidelines may not reflect a realistic standard of care in the real-world setting. While performing UPEP in addition to SFLC regularly may be the ideal practice for monitoring LCMM, impracticalities such as issues with collection technique and proper storage of urine samples may be hindering its regular use in the real world. The SFLC test may present a more realistic alternative for evaluation of disease status in patients with LCMM, and its ease of use could facilitate more frequent serial testing. This in turn could improve the measurement of progression at more regular intervals, potentially reducing patient exposure to therapies that are no longer effective. To our knowledge, this is the first study to investigate the differences in utilization of UPEP, SFLC, and SPEP testing conducted to follow MM and LCMM progression in the real world.
Foster:Flatiron Health: Employment. Lipitz:Flatiron Health: Employment. Torres:Flatiron Health: Employment. Carson:Roche: Consultancy; Flatiron Health: Employment; Washington University in St. Louis: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal